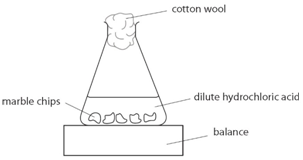
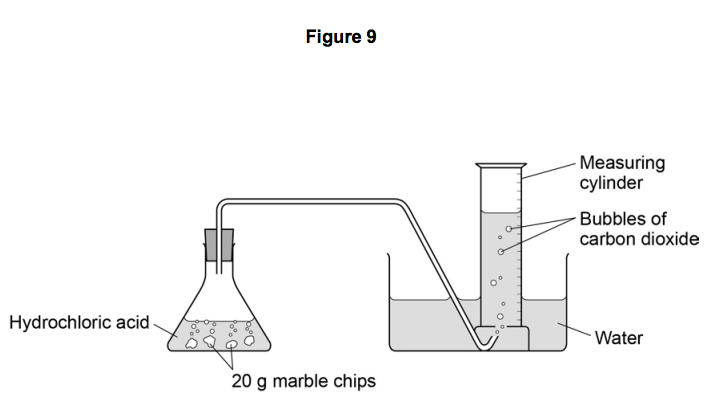
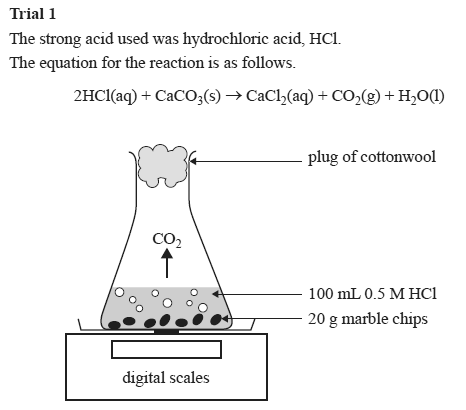
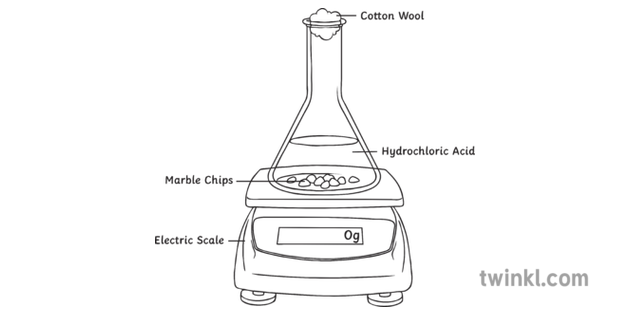
Marble chips and hydrochloric acid planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.
Marble chips and acid.
Describe how you could investigate.
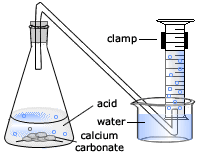
You then fill a bowl with water along with a boiling tube and straight after attach the delivery tube at the end of the boiling tube 4.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
Dilute sulphuric acid is made to react with marble chips 2 see answers mahfoozfarhan4 mahfoozfarhan4 so when calcium carbonate reacts with sulphuric acid it forms water carbon dioxide and calcium sulphate.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
Measuring the rate of reaction at various times.
First you measure out 25cm3 of hydrochloric acid 2.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Calcium carbonates which are marble chips react with hydrochloric acid to give out calcium chloride water and carbon dioxide.
Secondly weight out 3 grams of marble chips for each concentration 3.
With the equation caco3 2hcl cacl2 h2o co2 hypotheses a reaction occurs when particles collide.
The reaction between sulfuric acid and calcium carbonate is somewhat similar to the reaction with sodium bicarbonate way carbon dioxide.
Marble is calcium carbonate and thus behaves in the same hydrochloric acid calcium carbonate calcium chloride carbon dioxide water.
Pour the acid into the conical flask and add the marble chips 5.
The rate of this reaction can be changed by changing the size of the marble chips.